co2 electronic geometry|5.4.2: Carbon Dioxide : Clark CO 2 molecular geometry is based on a linear arrangement. The presence of a sigma bond and valence electron pairs repelling each other force them to move to the opposite side of the carbon atom, resulting in this geometric shape. As .
87 iyot cebu bisaya FREE videos found on XVIDEOS for this search.Täglich aktuelle Kenozahlen und Quoten der letzten Ziehungen und ein Archiv der KENO-Gewinnzahlen seit 2004
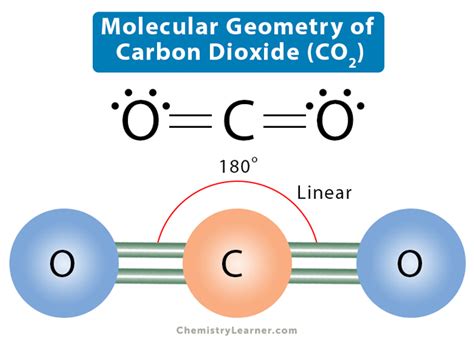
co2 electronic geometry,Hul 6, 2013 — A quick explanation of the molecular geometry of CO2 including a description of the CO2 bond angles. We can see that there are only two atoms attached to the central Carbon (C) atom and.Determine the Electron geometry from the Lewis dot structure. Determine the molecular geometry. It is very important from the onset that students understand the difference between electronic geometry and molecular geometry.5.4.2: Carbon Dioxide CO2 Geometry and Hybridization. First, we need to draw the Lewis structure of CO 2. In short, these are the steps you need to follow for drawing a Lewis structure: 1. Write the correct .
2 days ago — The electron geometry of CO2 is linear as well. Before you bombard me with questions about electron geometry, let me clear it out!! So molecular geometry is those which include only the atom while determining .
Ene 5, 2021 — In its electronic ground state, the carbon dioxide molecule has a linear geometry (Fig. 7.1) and belongs to the point group D ∞h. Both C-O bonds are equivalent with an .CO 2 molecular geometry is based on a linear arrangement. The presence of a sigma bond and valence electron pairs repelling each other force them to move to the opposite side of the carbon atom, resulting in this geometric shape. As .co2 electronic geometryWith knowledge of both orbital symmetries and energies, we can construct the molecular orbital diagram. The carbon atom goes on one side of the diagram while the oxygen SALCs are .The molecular geometry of CO2 can be determined using the valence shell electron pair repulsion (VSEPR) theory. According to this theory, the geometry of a molecule is .2 days ago — The electron geometry of CO2 is linear as well. Before you bombard me with questions about electron geometry, let me clear it out!! So molecular geometry is those which include only the atom while determining .Carbon dioxide has two electron groups and no lone pairs. Carbon dioxide is therefore linear in electron-group geometry and in molecular geometry. The shape of CO 2 is linear because there are no lone pairs affecting the orientation of the molecule. Therefore, the linear orientation minimizes the repulsion forces.
May 18, 2021 — Electron-pair Geometry versus Molecular Structure. It is important to note that electron-pair geometry around a central atom is not the same thing as its molecular structure. The electron-pair geometries shown in .Count the number of electron groups around each carbon, recognizing that in the VSEPR model, a multiple bond counts as a single group. Use Figure \(\PageIndex{3}\) to determine the molecular geometry around each carbon atom and then deduce the structure of the molecule as a whole.Ago 15, 2020 — Use Figure 9.3 to determine the molecular geometry around each carbon atom and then deduce the structure of the molecule as a whole. . To a first approximation, the VSEPR model assumes that multiple bonds and single bonds have the same effect on electron pair geometry and molecular geometry; in other words, VSEPR treats multiple bonds like .Moreover, the planer-shaped geometry, also called linear geometry, always has molecules with a 180-degree bond angle. CO2 Molecular Geometry. The concept of CO2 molecular geometry states that the molecular geometry of any compound depends on the arrangement of atoms, bonds, and electron pairs.Electronic Geometry, Molecular Shape, and Hybridization Page 1 The Valence Shell Electron Pair Repulsion Model (VSEPR Model) The guiding principle: Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. Bonded atoms Nonbonded Pairs Total Electronic Geometry Molecular
We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom, ignoring all other valence electrons present.According to this model, valence electrons in the Lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a lone pair of electrons, or .

Okt 27, 2022 — Electron-pair Geometry versus Molecular Structure. It is important to note that electron-pair geometry around a central atom is not the same thing as its molecular structure. The electron-pair geometries shown in Figure \(\PageIndex{3}\) describe all regions where electrons are located, bonds as well as lone pairs.Electron-pair Geometry versus Molecular Structure. It is important to note that electron-pair geometry around a central atom is not the same thing as its molecular structure. The electron-pair geometries shown in Figure 7.16 describe all regions where electrons are located, bonds as well as lone pairs. Molecular structure describes the location of the atoms, not the electrons.Carbon Dioxide: Carbon dioxide is a chemical compound made when carbon combines with oxygen in a 1:2 ratio. It is a gas at room temperature and pressure and it is environmentally significant as a driver of climate change.
Hun 10, 2019 — The electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{CH_4}\). In the ammonia molecule, one of the electron pairs is a lone pair rather than a bonding pair. Although the lone pair is not visible, it will affects the location and bond angles among other atoms in the molecule.

Therefore, it has a tetrahedral electron geometry and a bent molecular geometry: Carbon 4 is connected to three atoms, and no lone pairs. SN = 3, which corresponds to a trigonal planar electron and molecular geometry. .
Okt 10, 2023 — Carbon disulfide is made up of one carbon and two sulfur having the chemical formula CS2. It is a neurotoxic colorless volatile liquid. In this article, we will discuss Carbon disulfide (CS2) lewis structure, molecular or electron geometry, bond angle, hybridization, polar or nonpolar, etc.18 hours ago — Now, in NO2 we have 17 valence electrons, therefore this is an odd-electron system. For single electron species, we have the following rule to follow: If the oxidation state of the central atom is found positive then the electron will participate in the hybridization process but if the oxidation state is negative, it will not participate.co2 electronic geometry 5.4.2: Carbon Dioxide Ene 24, 2021 — In this video we look at the electron geometry for CO (Carbon Monoxide). Because the Carbon Monoxide molecule has two electron domains (one oxygen atoms and .18 hours ago — The atomic number of carbon is 6, where its electronic configuration is 1s2 2s2 2p2. To achieve a stable state, the p shell needs to accommodate 6 valence electrons. So, the total number of valence electrons in carbon is 4. . Molecular Geometry of Carbonyl Fluoride (COF2) Molecular geometry is a 3D diagrammatic way of studying the structure .Dis 12, 2020 — Thus, CO2 has a linear molecular geometry. I) Electron Domain (ED) Geometry. From the above Lewis dot structure, CO2 has only two regions of electron density around the central carbon atom because no lone pair of electrons presence of carbon atom. . The molecular geometry of CO2 is linear with a bond angle of 180 ° because the dipole charges .
co2 electronic geometry|5.4.2: Carbon Dioxide
PH0 · Properties of the Carbon Dioxide Molecule
PH1 · Electron Geometry for CO2 (Carbon Dioxide)
PH2 · CO2 Molecular Geometry and Bond Angles (Carbon Dioxide)
PH3 · CO2 Lewis Structure, Molecular Geometry and Hybridization
PH4 · CO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagr
PH5 · CO2 Lewis Structure, Hybridization, Molecular
PH6 · CO2 Lewis Structure, Geometry
PH7 · CO2 Lewis Structure,
PH8 · CO2 Geometry and Hybridization
PH9 · 8.6: Molecular Geometries
PH10 · 5.4.2: Carbon Dioxide